Background: Ineffective erythropoiesis and anemia are pathognomonic features of β-thalassemia, leading to red blood cell (RBC) transfusion dependence in patients with more severe genotypes and consequently increased morbidity and mortality. The phase 3 BELIEVE study showed that luspatercept reduces transfusion burden in transfusion-dependent patients with a manageable safety profile (Cappellini MD, et al. HemaSphere 2022;6[S3]:171-172; Viprakasit V, et al. HemaSphere 2022;6[S3]:2664-2665). Patients who continued to benefit from luspatercept at completion of the BELIEVE study or who needed to complete post-treatment follow-up could roll over to the phase 3b long-term follow-up study (LTFU; NCT04064060) to continue receiving treatment or complete post-treatment follow-up, respectively. Patients receiving luspatercept were monitored for continued efficacy and safety.
Methods: The aim of this analysis was to evaluate long-term safety and efficacy of luspatercept in patients who rolled over to the LTFU study. This analysis included patients randomized to luspatercept during BELIEVE (luspatercept group) and those randomized to placebo who crossed over to receive luspatercept after unblinding (crossover group). Response was defined as ≥ 33% reduction from baseline in RBC transfusion burden during any 12-week interval over the entire treatment period of the combined studies. The data cutoff was Jan 2, 2023.
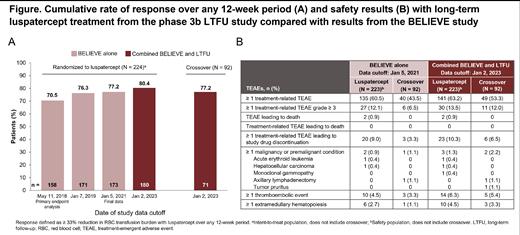
Results: At the closure of the BELIEVE trial, 127/224 (56.7%) patients from the luspatercept group and 67/92 (72.8%) patients from the crossover group had transitioned to the LTFU study with the intention to continue luspatercept. At data cutoff, treatment was ongoing for 138 patients, 90 in the luspatercept group and 48 in the crossover group. Median (range) total treatment duration over the combined studies was 229.1 (1.7-336.1) weeks for the luspatercept group and 221.2 (6.1-230.7) weeks for the crossover group; 98 patients had completed 288 weeks (> 5 years) of treatment at data cutoff, all of whom had been randomized to luspatercept.
In the luspatercept group, 180/224 (80.4%) patients had achieved response, continuing the trend showing an increasing proportion of responders with longer duration of treatment observed at previous data cutoffs (Figure A). Response in the crossover group was similar with 71/92 (77.2%) responders. Patients in the luspatercept and crossover groups had median (range) reductions in transfusion burden of −17.75% (−100.0 to 33.3) and −19.05% (−100.0 to 20.8), respectively, from baseline to week 145-192, equating to mean reductions of 6.78 RBC units (SD 7.27; n = 120) and 6.85 RBC units (SD 6.78; n = 50), respectively.
Liver iron concentration in the luspatercept group was reduced with long-term treatment at each timepoint compared with the baseline (mean decrease of 2.80 mg/g dry weight [dw] at week 144 from median 8.97 [range, 1.1-31.9] at baseline [n = 24]; mean decrease of 0.45 mg/g dw at week 192 from median 7.75 [1.4-35.5] at baseline [n = 27]; mean decrease of 1.48 mg/g dw at week 240 from median 5.65 [1.0-42.0] at baseline [n = 20]). Mean changes from baseline in overall serum ferritin levels (last 24-week level for each patient prior to their efficacy cutoff) were −376.27 μg/L for luspatercept and +43.09 μg/L for crossover.
In the combined studies up to data cutoff, 59.4% of luspatercept patients and 47.8% of crossover patients discontinued treatment. The most frequent reason for treatment discontinuation was the patient's personal decision to withdraw (luspatercept: 23.7% in BELIEVE and 33.5% in the combined studies; crossover: 14.1% in BELIEVE and 28.3% in the combined). Discontinuation due to an adverse event (AE) in the combined studies was similar to experience from BELIEVE (luspatercept: 10.3% BELIEVE and 12.5% combined; crossover: 4.3% BELIEVE and 7.6% combined). One patient (0.4%) discontinued luspatercept and transitioned to a commercially available treatment. The frequency of treatment-emergent AEs in the combined studies was also consistent with previous experience from the BELIEVE trial (Figure B).
Summary: These long-term data from the LTFU study show that erythroid responses and safety results in both the luspatercept and crossover groups were consistent with previous clinical experience from the BELIEVE trial. Patients receiving long-term luspatercept treatment experience durable clinical benefit with no new safety concerns.
Disclosures
Cappellini:Bristol Myers Squibb: Honoraria, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Vertex: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Research Funding; Silence Therapeutics: Membership on an entity's Board of Directors or advisory committees. Taher:Pharmacosmos: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Vifor: Consultancy, Research Funding; Bristol Myers Squibb (Celgene): Consultancy, Research Funding; Novartis Pharmaceuticals: Consultancy, Research Funding. Viprakasit:BMS: Research Funding; Novartis: Research Funding; Silence Co. Ltd.: Research Funding; GPO, Thailand: Research Funding. Georgiev:UMHAT St George: Current Employment; Medical University Plovdiv: Current Employment; Bristol Myers Squibb: Other: Speaker; Novartis: Other: Speaker; Roche: Other: Speaker. Kuo:Agios Pharmaceuticals: Consultancy, Research Funding; Alexion Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; Forma Therapeutics: Consultancy; Pfizer: Consultancy; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Sangamo: Membership on an entity's Board of Directors or advisory committees; Novo/Nordisk: Consultancy, Honoraria; Vertex Pharmaceuticals: Consultancy. Holot:Bristol Myers Squibb: Current Employment. Vilmont:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Felber Medlin:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Kuo:Bristol Myers Squibb: Current Employment. Lai:Bristol Myers Squibb: Current Employment. Moro Bueno:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Sheth:Fulcrum: Consultancy; Bluebird bio: Consultancy, Other: Travel support; Bristol Myers Squibb/ Celegene: Consultancy, Other: Travel support, Research Funding; Chiesi: Consultancy; CRISPR: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Other: Travel support, Research Funding; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.